Functional Botanicals
Nutritional Products
We delivers superior functional botanicals & supplement manufacturing services through our GMP-certified facilities and scientifically-validated processes. We specialize in developing and producing high-potency nutritional supplements with exacting quality standards and comprehensive regulatory compliance. Our integrated approach encompasses formulation development, raw material sourcing, precision manufacturing, and quality assurance, ensuring market-ready products that meet stringent international standards.
Key Benefits
US FDA and WHO-GMP compliant manufacturing facilities
98.7% production accuracy rate across all formulations
30-day standard turnaround for most product categories
Full documentation support for global regulatory submissions
R&D support for novel ingredient applications
Send Enquiry
Formulation Expertise & Development
Scientific Formulation Development
Evidence-based formulation with bioavailability enhancement
Our formulation team combines nutritional science with manufacturing expertise to develop effective products that meet specific health objectives and market requirements. characteristics.
Scientific Formulation

Development Capabilities
Bioavailability Optimization : Enhanced absorption technologies including liposomal delivery and nanoemulsion
Synergistic Blending : Scientifically-validated ingredient combinations for maximum efficacy
Stability Testing : Accelerated and real-time stability studies per ICH guidelines
Compatibility Analysis : Ingredient interaction studies and excipient compatibility testing
Formulation Portfolio
Vitamins and minerals in various forms and potencies
Herbal and botanical extracts with standardized active compounds
Sports nutrition and performance supplements
Specialized health condition formulations
Custom blends for specific demographic needs
Quality Assurance & Regulatory Compliance
GMP Manufacturing Standards
Potent grade quality management system
Our manufacturing facilities operate under strict Good Manufacturing Practice guidelines with comprehensive documentation and quality control protocols.
Quality Control Protocols
Raw material identity testing through HPLC and GC-MS
In-process quality checks at critical control points
Finished product comprehensive testing
Stability studies for shelf-life determination
Microbial testing: Total plate count, yeast/mold, pathogens
Quality Framework
Facility Certification : US FDA registered, WHO-GMP compliant, ISO 9001:2015 certified
Documentation System : Complete batch records, SOPs, and quality manuals
Personnel Training : Certified technicians and continuous GMP training programs
Facility Design : Class 100,000 clean rooms with environmental monitoring
GMP Standard

Global Regulatory Compliance
Market-Specific Expertise
Comprehensive regulatory support for international markets
We provide complete regulatory guidance and documentation support for registration and compliance in major global markets.
Compliance Capabilities
US Market : FDA dietary supplement regulations, DSHEA compliance, structure/function claim guidance
EU Market : Food supplement directive compliance, novel food applications, health claim regulations
Canada : Natural Health Product Regulations, product licensing, site licensing
Australia : TGA complementary medicines regulation, listed medicine registration
Other Markets : Country-specific regulatory support and documentation
Documentation Services
Certificate of Analysis with full specification testing
Stability study reports and shelf-life justification
GMP certificates and manufacturing compliance statements
Allergen statements and religious certifications (Kosher)
Export documentation and certificates of free sale
Regulatory Compliance

Manufacturing Process Excellence
Raw Material Management
Raw materials sourcing and testing
Our raw material procurement process ensures the highest quality ingredients with complete traceability and documentation.
Material Standards
Supplier Qualification : Comprehensive vendor qualification program
Identity Testing : HPLC, GC-MS, FTIR for ingredient verification
Purity Testing : Heavy metals, pesticides, microbial contaminants
Potency Verification : Assay testing for active ingredient quantification
Documentation : Certificate of Analysis, material safety data sheets
Quality Metrics
100% identity testing on all raw materials
<0.5% material rejection rate
Real-time supplier performance monitoring
24-month material traceability retention
Traceability

Production Process Control
Raw Material Management
Precision manufacturing with automated process controls
Our manufacturing processes incorporate advanced technology and rigorous controls to ensure product consistency and quality.
Process Capabilities
Weighing & Dispensing : Automated weighing systems with ±0.5% accuracy
Mixing & Blending : High-shear mixers with homogeneity validation
Granulation : Fluid bed and high-shear granulation with moisture control
Compression : Rotary tablet presses with force monitoring and weight control
Encapsulation : Automatic capsule fillers with weight variation control
Coating : Film coating systems with uniform coating application
Process Validation
Installation Qualification (IQ) for all equipment
Operational Qualification (OQ) for process parameters
Performance Qualification (PQ) for product-specific validation
Ongoing process verification and control chart monitoring
Packaging & Labelling Solutions
Primary Packaging Options
Compliant packaging with product protection features
We offer comprehensive packaging solutions that ensure product stability, compliance, and market appeal.
Packaging Formats
Bottles : PET, HDPE, glass with child-resistant closures
Blister packs : PVC, PVDC, aluminium foils for moisture protection
Pouches : Stand-up pouches with zip locks and moisture barriers
Sachets : Single-dose and multi-dose foil sachets
Custom packaging : Brand-specific packaging development
Packaging Validation
Stability testing in final packaging
Compatibility studies with packaging materials
Child-resistant testing per ISO standards
Moisture protection validation
Light protection testing
Packaging Option

Labelling Compliance
Primary Packaging Options
Market-specific labelling requirements
Our labelling services ensure complete compliance with destination market regulations while maintaining brand identity.
Labelling Services
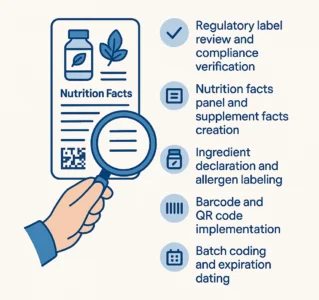
Regulatory label review and compliance verification
Nutrition facts panel and supplement facts creation
Ingredient declaration and allergen labeling
Multilingual labeling for international markets
Barcode and QR code implementation
Batch coding and expiration dating
Compliance Features
100% regulatory compliance guarantee
48-hour label review and approval process
Digital proofing and approval system
Label claim substantiation support
Legal review for structure/function claims
Labelling Compliance
Technical Support Services
Research & Development
Continuous product development and improvement
Our R&D team provides comprehensive support for new product development and existing product optimization.
R&D Services
New ingredient evaluation and validation
Formulation development and optimization
Process development and scale-up
Analytical method development and validation
Stability study design and execution
Innovation Metrics
15 new formulations developed monthly
12-month product development timeline
95% first-time formulation success rate
Continuous improvement program implementation
Analytical Testing Services
Laboratory Capabilities
Comprehensive in-house testing facilities
Our analytical laboratory provides complete testing services to support product development and quality control.
Testing Capabilities
HPLC : Potency testing, dissolution testing, related substances
GC-MS : Residual solvent testing, essential oil analysis
ICP-MS : Heavy metal testing and mineral analysis
Microbiology : Microbial limits testing, pathogen detection
Physical Testing : Disintegration, dissolution, hardness, friability
Quality Metrics
99.8% testing accuracy rate
24-hour turnaround for routine testing
48-hour method development capability
Continuous method validation and verification
In-House Reports